You can see it coming in right there, that little spot,” says neuroscientist and engineer Laura Lewis.
A remarkably bright pulsing dot has appeared on the monitor in front of us. We are watching, in real time, the brain activity of a graduate student named Nick, who is having an afternoon nap inside an imaging machine at the Massachusetts Institute of Technology, where Lewis has her laboratory.
The bright spot first appears toward the bottom of the screen, about where Nick’s throat meets his jaw. It moves slowly upward, fades and then is followed by another bright dot. “It really comes and goes,” says Lewis, who is also affiliated with Massachusetts General Hospital. “It’s in waves.” This moving dot depicts something few people have ever seen: fresh cerebrospinal fluid flowing from the spinal cord into the brain, part of a process that researchers are now learning is vital for keeping us healthy.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
For decades biologists have pondered a basic problem. As human brains whir and wonder throughout the day, they generate waste—excess proteins and other molecules that can be toxic if not removed. Among those proteins are amyloid beta and tau, key drivers of Alzheimer’s disease. Until recently, it was entirely unclear how the brain takes out this potentially neurotoxic trash.
In the rest of the body, garbage removal is handled initially by the lymphatic system. Excess fluid and the waste it carries move from tissue into the spleen, lymph nodes and other parts of the system, where certain particles are removed and put into the bloodstream to be excreted. It was long thought that the brain can’t use the same trick, because the so-called blood-brain barrier, a protective border that keeps infections from reaching critical neural circuitry, stops the transport of most everything in and out.
In 2012 researchers at the University of Rochester led by neuroscientist Maiken Nedergaard made a pivotal discovery: a previously unknown circulatory system was flushing toxic waste from the brain. In mice, they showed that an influx of cerebrospinal fluid (CSF) washes through the brain’s “perivascular” spaces, which are doughnut-shaped tunnels that surround blood vessels. Using water channels on the surface of astrocytes, a type of cell that supports brain function, the CSF mixes with “interstitial” fluid in the spaces around the brain’s cells and collects built-up waste. Then the fluid leaves the brain through the perivascular spaces around veins, taking the garbage with it.
Nedergaard and her team called their discovery the glymphatic system—“g” for glial cells, of which astrocytes are a subtype, and “lymphatic” to reference the waste-clearance function. The next year, in 2013, they published an important additional finding: this housekeeping was most active and efficient during sleep. “Wakefulness clearly shut it down,” Nedergaard says—probably because the precision that neural networks need to process the external world when awake isn’t compatible with the clean-up process. That finding suggests this newly discovered brain-washing process is one of the critical functions of sleep. “Sleep is clearly for the brain,” Nedergaard says. “When you wake up refreshed after good sleep, it is probably because your brain had a tune-up similar to your car.”
But this groundbreaking work was done in mice, and mice are not people. Their brains are smaller and less complex than ours, their sleep far more fragmented. In part because of that discrepancy, the glymphatic hypothesis has had plenty of naysayers. “Ten years ago all this flow in the brain, it was almost like heresy,” says neuroimmunologist Jonathan Kipnis of the Washington University School of Medicine in St. Louis.
Hundreds of studies have since been done—and that bright dot Lewis showed me represents a critical next phase of investigation. She and others have spent much of the past decade exploring whether this waste-clearance process works in humans as it does in rodents. The short answer seems to be yes. Furthermore, the electrical waves that sweep through the brain during sleep, helping to sort, select, transport and store memories, seem to have another significant function: they also propel cerebrospinal fluid in and out of the brain.
The significance of the glymphatic system is considerable. If waste clearance is an essential function of human sleep, then a dysfunction in this system probably relates to many neurological and psychiatric disorders, including Alzheimer’s. Glymphatic impairment could explain why the aging brain accumulates the amyloid plaques and tau tangles that trigger Alzheimer’s—and there is some evidence that conditions such as traumatic brain injury, which is associated with Alzheimer’s, interfere with waste clearance. “If it’s the thing that holds all those things together,” says Jeffrey Iliff, a professor of psychiatry and neurology at the University of Washington School of Medicine who worked with Nedergaard on the original studies, “well then if you target it, that opens the door to primary prevention of neurodegenerative diseases.”
Although it’s been clear for years that accumulation of amyloid and tau proteins leads to Alzheimer’s, the link between sleep and the waste-clearance process that might get rid of them was not obvious. For decades sleep researchers focused primarily on sleep’s role in memory processing. For their part, biologists who studied the blood-brain barrier knew there were perivascular spaces surrounding blood vessels like loose sheaths, but they didn’t know what purpose those spaces served and largely discounted the idea that they were conduits for fluids, Iliff says. “They didn’t see how dynamic it was.”
Early on, Nedergaard and Iliff, a glial cell biologist and a vascular physiologist, respectively, hypothesized that waste clearance might actually require wakefulness. They mistakenly reasoned that the brain is less active during sleep and, therefore, that glymphatic function would be lower at night.
David Cheney; Source: Illustration by N. Desai in “Deep Sleep Drives Brain Fluid Oscillations,” by Søren Grubb and Martin Lauritzen, in Science, Vol. 366; November 2019 (primary content reference)
In truth, the brain is not less active during sleep; it is differently active. Traditional sleep studies use electroencephalography (EEG) to track electrical activity that aligns with the phases of sleep. Their findings show that while people are awake and alert, patterns of neural activity are fast, characterized by high-frequency waves. In the early, light phases of sleep, known as stage 1 and stage 2, activity slows down and low-frequency waves appear. Deep sleep, or stage 3, is known as slow-wave sleep for the high-amplitude, lowest-frequency “delta” waves that dominate it. These waves, which on an EEG readout look like chains of large ocean swells, help the brain sort through the day’s experiences and store some of them as memories. In contrast, the fourth phase, called rapid eye movement (REM) sleep because the eyes flit quickly from side to side beneath the eyelids, is when we have our most vivid dreams. The period resembles wakefulness, with faster brain activity than in other sleep stages.
What EEG cannot detect is the flow of fluids in the brain, however. Every day our bodies produce and then drain three to four times the volume of cerebrospinal fluid that we have. The few early studies of the fluid using magnetic resonance imaging (MRI) recognized that its flow was coupled to heartbeats but couldn’t go further because the technology wasn’t up to the challenge. There was also little awareness that CSF flow changed during sleep.
The first real sign of sleep’s importance in waste clearance was Nedergaard’s pioneering 2013 study. It compared the clearance of amyloid beta proteins from the brain in mice that were awake, sleeping or anesthetized. The researchers injected a fluorescent tracer into mouse brains and found its influx into perivascular spaces and brain tissue was reduced by an astonishing 95 percent when mice were awake compared with when they were sleeping. The volume of the interstitial space in the brain’s cortex also increased by 60 percent when the mice were asleep or anesthetized, suggesting that sleep led to physiological changes designed to increase the brain’s ability to get rid of waste. Ultimately amyloid beta moved out of the brains of sleeping mice twice as fast as in mice that were awake.
Would it work the same way in humans? That was the question that neurosurgeon and researcher Per Kristian Eide asked. He was studying the relation between glial cells such as astrocytes and the dense network of blood vessels in the human brain at Oslo University Hospital in Norway. As a surgeon, Eide could take advantage of the fact that he was already working inside people’s heads and—with permission—do some extra research.
With radiologist Geir Ringstad and others, Eide launched a study, published in 2021, with patients who were already undergoing neurological assessment in the hospital. The scientists injected a tracer into the CSF of all participants. One group was allowed to sleep normally through the night; the other was kept awake for 24 hours. All participants underwent multiple MRI scans in the evening and again the next day.
Removal of the tracer was dramatically slower in those who had not slept, compared with those who had. “It was very evident,” Eide says. “We were very, very surprised that we saw something after one night of sleep deprivation.” Even more notable was that after all participants were allowed to sleep normally the next night, the clearance of the tracer was still slower in those who had lost the earlier night of sleep. “You don’t compensate by having a good night’s sleep,” Eide says.
In a subsequent study, Eide and his team found that people who reported chronic poor sleep also showed delayed clearance of the tracer. Moreover, in people with dementia, brain volumes in the frontal, temporal and parietal lobes had shrunk compared with those who slept well. In part because dementia has been previously associated with poor sleep quality, perhaps because of atrophy in the cortex, Eide and his colleagues suspect that chronic sleep disturbance co-occurs with glymphatic dysfunction.
There were also clear differences between humans and mice. In the rodents, for example, glymphatic transport was “an on-off phenomenon,” Eide says—on during sleep and off when the mice were awake. In humans, the process is not as extreme, and changes occur over hours rather than minutes. The work nevertheless demonstrated that human brains, too, get cleaned during sleep—and that “poor sleep quality is affecting your glymphatic function,” as Eide says.
Laura Lewis has lost a lot of sleep in the course of her work because she conducts all-night experiments. “The sad irony of being a sleep researcher is that you can’t follow your own advice,” she says. Lewis has tackled a different piece of the glymphatic problem: the movement of fluids in the human brain that underpin waste clearance. That’s how, about seven years ago, she came to be in the control room of an MRI machine very like the one in which she showed me Nick’s CSF.
Lewis had chosen to measure CSF flow in the brain’s fourth ventricle, a small cavity tucked against the cerebellum at the base of the brain. The ventricles produce CSF and act like extra shock absorbers for the fragile brain. For Lewis’s purposes, the fourth ventricle was useful because “it’s a kind of choke point.” Sitting as it does at the base of the brain, it provides a summary of what is happening elsewhere—like measuring attendance in a crowded room as people come through the door.
In an overnight sleep study published in 2019, Lewis and her colleagues were the first to use MRI to view this process in action. By taking pictures of the brain every 367 milliseconds instead of the standard two or three seconds, they were able to see the movement of cerebrospinal fluid during sleep.
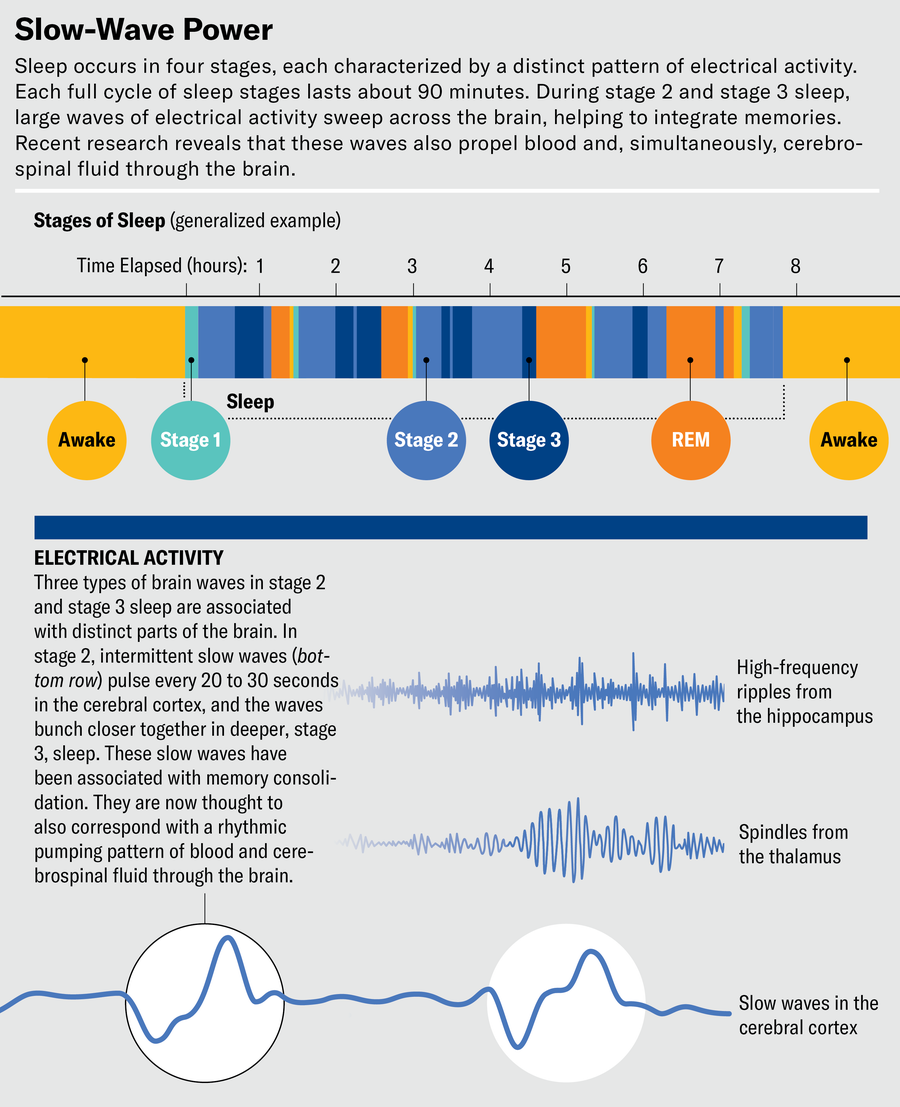
At first, Lewis couldn’t quite believe it. Typically the significant details of brain imaging require statistics and processing to tease out; they aren’t something you can see by eye. “It was honestly the biggest signal I’ve ever seen,” Lewis says. “It was crazy. It’s really striking how much you can see that this is happening during sleep.”
Lewis delayed publishing the research until she had triple-checked it. It has since been replicated several times. “When people are sleeping, there are these really huge and slow waves of flow that are pulsing every 20 to 30 seconds in the brain, specifically when we’re in non–rapid eye movement sleep,” Lewis says. Using EEG data, she also saw clear patterns of brain activity before each wave. As delta waves of deep sleep and, to some extent, “theta” waves during intermediate (stage 2) sleep sweep through the brain, transmitting and integrating memories, they also seem to propel pulses of CSF into the brain.
Lewis found that CSF also flows when people are awake but less effectively. “It’s always moving a little, but then when you fall asleep, a new cycle starts,” she says. The difference in fluid movement is like the change when a washing machine switches from light jiggling to full-on rotation, at which point more water pours in and out. Lewis’s conclusion: sleep, a state that is essential for human health, has a distinct pattern of CSF flow—and that pattern changes as the stages of sleep shift. “It’s not a coincidence,” she says. “It’s actually the same brain circuits that are controlling sleep that seem to also be engaged and controlling the flow.”
But what is the source of the elbow grease necessary to do the cleaning? Unlike the blood, which is pumped through the body with great force by the heart, the cerebrospinal fluid is more like water in a bathtub or a slow-moving river with many tributaries. “Where is the force coming from?” Lewis asks.
One possibility is the neurotransmitter norepinephrine, also called noradrenaline. Norepinephrine surges when we wake up. During the day, it focuses attention; it also works to constrict blood vessels. And during non-REM sleep, according to a new study of mice by Nedergaard’s group, a lower level of norepinephrine release—not enough to wake the mice but enough to make their blood vessels pulse—propels the movement necessary for CSF to flow.
The blood vessels in the brains of these mice dilated and constricted with an amplitude that was 10 times larger during non-REM sleep than during wakefulness, Nedergaard says. And as the blood vessels wax and wane, pushing blood in and out of the brain, CSF flows in and out to fill the expanding and contracting spaces around the blood vessels. “It seems to be this chain of events where your sleep state changes, and then that changes your blood vessels, and those actively pump the flow of CSF in the brain,” Lewis says. In this way, oscillations in norepinephrine cause waves of CSF to pulse through the perivascular sheaths.
But norepinephrine isn’t the whole story. In February 2024 Kipnis, neuroscientist Li-Feng Jiang-Xie, and their colleagues at Washington University published a paper showing that, ultimately, it’s neurons that provide the energy necessary for cleaning. “A neuron is a tiny little pump,” says Jiang-Xie, who is now at the University of Pennsylvania. The electrical activity of synchronized neurons, especially during sleep, can propel fluid flow through the brain tissue and help clean waste out. That idea was implied in Lewis’s earlier study, but by working in mice, Jiang-Xie and Kipnis were able to show in detail how fluid moved in and out of brain tissue. “Norepinephrine is basically controlled by neurons’ activity,” Jiang-Xie says. These results are “beautifully aligned with what we found in humans but provide information we couldn’t have gotten,” Lewis adds.
Meanwhile other research in mice has revealed important distinctions between natural sleep and anesthesia, as well as among anesthetics, all of which affect waste clearance differently. An anesthetized brain does not cycle through phases as it does in sleep. And it turns out that some anesthetics suppress glymphatic function, whereas others enhance it. Furthermore, the influx of cerebrospinal fluid occurs in direct proportion to the power of slow-wave neural activity and in inverse proportion to heart rate, both of which are affected by the drugs used. Those findings help to explain some studies that haven’t supported the glymphatic theory. For instance, a 2017 report from researchers at the University of California, San Francisco, described mice anesthetized with avertin, which has since been shown to limit how much cleaning the glymphatic system can accomplish. Five other labs have reconfirmed the initial finding that glymphatic clearance works during sleep.
Where does the fluid go? That has been another persistent question. The brain-washing process is made up of four stages, Kipnis says. There is CSF flow into the brain, within the brain, out of the brain along the veins, then into the lymphatic system. His focus has been on the last of these, which is as consequential as everything that comes before it. “If you wash your house with the same bucket of water, it will not be washing; it will be moving dirt from one place to another,” Kipnis says.
The solution is to empty the bucket and bring a new one, which means, in the brain, ensuring that the lymphatic vessels into which the “dirt” will be dumped are functioning. In 2015 Kipnis and his colleagues found the sewage system: they reported the discovery of lymphatic vessels in the meninges that envelop the brain and spinal cord. These vessels represent an important missing piece of the glymphatic puzzle because they can receive cerebrospinal fluid and interstitial fluid from the brain. They are “the final outpost,” Kipnis says. “There is a biological structure at the very end of the whole process.”
Once scientists can work out precisely how glymphatic clearance works, they should also begin to see how things go wrong when it doesn’t work. Norepinephrine and sleep disruption, for example, are also involved in the development of chronic pain. Higher bursts of norepinephrine lead to wakefulness and seem to shut off the glymphatic system. In consequence, improving the system by reducing norepinephrine levels could possibly also reduce chronic pain. Nedergaard is also exploring the glymphatic system’s possible role in psychiatric disorders such as depression and schizophrenia.
Eide wants to enhance glymphatic function. The cerebrospinal fluid is an appealing route to deliver drugs to the brain because it bypasses the blood-brain barrier. And the role of norepinephrine is exciting, Nedergaard says. Whether there is too little norepinephrine signaling, as in Alzheimer’s, or too much, as in chronic pain or stress, the brain-wave oscillations it controls become inefficient. That recognition might enable us to target treatment by modulating norepinephrine in drug form.
There are limits to the possibilities. And until there is a drug that enhances the glymphatic system as well as amyloid beta and tau clearance and slows progression of pathology, no one can definitively say that impaired clearance of brain toxins causes Alzheimer’s in humans. In familial early-onset Alzheimer’s, amyloid proteins are produced in excess, and clearance may not be able to keep up, no matter how much it is improved. Yet even if enhancing waste clearance only slows the development of Alzheimer’s for most patients, that is a big deal. Being able to enjoy five to eight more years of living free of impairment would be a game changer.
There may be nonpharmaceutical strategies, too. In the study for which Nick took an afternoon nap, graduate student Joshua Levitt is experimenting with sound stimuli while people are sleeping. His research combines EEG and functional MRI to capture sleep state and brain activity. With Nick and others, he’s sending staticky beeps into headphones while subjects are asleep. Given that CSF tends to flow in slow waves and, moreover, that sounds cause more slow-wave activity, this strategy could theoretically affect cerebrospinal flow. “If we think these things are relevant for great health, then we need to develop ways to actually change them,” Lewis says. Levitt “is trying to potentially enhance sleep.”
Simply understanding the flow of cerebrospinal fluid and how it changes with age is also important. Another graduate student in Lewis’s lab, Sydney Bailes, is investigating the differences in flow between adults older than 60 and younger than 40 and the potential implications for waste clearance. It’s normal to sleep less as you age and to have fewer slow waves. “How can we separate just a typical age-related change in sleep versus one that’s starting to become an impairment?” Lewis asks. “We need to disentangle those.”
That study is still underway, but so far they have found that older individuals differ from one another more than the younger group does—a pattern that mimics the wider range of cognitive differences in older people versus younger people. “You’ll see some measures that are very clustered together for the young adults and very spread out for the older adults,” Bailes says.
But people can surprise. An 80-year-old woman, one of the oldest in the study, stood out. “Her waves have a much larger amplitude than I typically see in older adults, and they seem to be also pretty consistent,” Bailes says. “Her CSF flow looked like a young person’s CSF.”
That’s a striking statement. Could it someday become a routine way of evaluating a person’s health? Quite possibly. Multiple labs are working toward a noninvasive “glymphogram” that would reveal how well a person’s clearance system is working. Glymphatic function may someday be like hypertension, something to be treated before it turns into a more serious condition. Certainly differences in waste clearance could help explain why some people age healthily and others do not—and then it could pave the way to treatments that enhance clearance in those who need it. The goal is not for everyone to sleep like a baby; sleeping like a thirtysomething would do.

