Cell lines
HEK293T, HeLa and U2OS cells were purchased from ATCC. All cell lines were authenticated by short tandem repeat analysis performed at Mayo Clinic. Cell lines were routinely tested for Mycoplasma contamination. HEK293T and HeLa cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM). U2OS cells were cultured in DMEM:F12 supplemented with 10% FBS. All cells were grown at 37 °C with 5% CO2.
Plasmid transfection and lentiviral infection
Full-length KCTD10 and the IDR2Δ deletion were cloned into pLVX3-CMV-puro (3×Flag at the N terminus) vector using PCR amplification of cDNA with restriction sites added as a 5′ primer overhang. BTBΔ, PIP3A and PIPΔ mutants of KCTD10 were generated by site-directed mutagenesis of the full-length KCTD10-pLVX3 plasmid (Q5 site-directed mutagenesis, New England Biolabs). KCTD10 shRNAs and TOP2B shRNAs were purchased from Sigma-Aldrich. Scramble shRNA was a gift from D. Sabatini56. MYC–CUL3 (pcDNA3-MYC-CUL3) was a gift from Y. Xiong57. TCEA1, TCEA2 and TCEA3 cDNA was synthesized (Genewiz), digested and ligated into pLVX3-CMV-puro.
Cells were transfected with TransIT-X2 (Mirus) according to manufacturer’s instructions. For transient expression, cells were analysed 48 h after transfection. To generate lentivirus, HEK293T cells were transfected with the plasmid of interest, psPAX2 and pMD2G (at a ratio of 5:3.75:1.25). Thirty-six to forty hours after transfection, culture supernatants were collected, centrifuged at 800g for 5 min and passed through a 0.2-μm filter to remove cells and debris. Filtered virus was then split into 1 ml aliquots and stored at −80 °C until use.
For lentiviral transduction, cells were seeded in one well of a 6-well plate 16–18 h prior to infection. At the time of infection, 1 ml of medium was removed and replaced with 1 ml of viral supernatant together with 10 μg ml−1 polybrene. For experiments with transient knockdown or expression, cells were used 48 h later for experiments. For stable cell lines, cells were selected with the appropriate antibiotic for at least one week. Stable cell lines were frozen at this stage and not used for more than 15 passages after this point.
Immunoprecipitation and immunoblotting
Cells were washed three times with PBS and lysed in NETN buffer (20 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, supplemented with 50 mM β-glycerophosphate, 10 mM NaF and protease inhibitors) at 4 °C with rotation for 30 min. Cell lysates were clarified by sonication with a Bioruptor Plus (Diagenode) for 8 cycles (30 s ON/30 s OFF, at 4 °C) and centrifuged at 12,500g for 15 min. For immunoprecipitations, clarified lysates were incubated with anti-Flag M2 Affinity Gel (Sigma) or with protein A/G magnetic beads (ThermoFisher) for 2 h at 4 °C with rotation. Beads were washed four times with lysis buffer. For SDS–PAGE, clarified lysates were then diluted into Laemmli buffer and boiled for 5–10 min at 95 °C. Samples were migrated through stacking gels at 75 V and resolving gels at 200 V using Tris-glycine-SDS buffer. Wet transfers were performed for 60 min at 300 mA using Tris-glycine buffer. Membranes for phosphoprotein blots were blocked in 5% BSA in TBST, and all other membranes were blocked in 5% fat-free milk in TBST for 30 min at room temperature. Membranes were incubated with primary antibody solutions in TBST overnight at 4 °C and in secondary antibody solutions in TBST for 1 h at room temperature. Membranes were imaged using SuperSignal West Pico PLUS Chemiluminescent Substrate (ThermoFisher) on a LI-COR Odyssey Fc.
Size-exclusion chromatography
Size-exclusion chromatography was performed as described58. In brief, cells were treated with ICRF193 or control (DMSO). Cell lysates were run on an AKTA Purifier FPLC system with a Superdex 200 gel filtration column (GE Healthcare) to separate different fractions. Collected fractions were subjected to western blotting and the indicated fractions were subsequently incubated with anti-KCTD10 antibody (Sigma) overnight. The immunocomplexes were then separated by SDS–PAGE and western blotting was done as described above.
Denaturing Ni-NTA pulldowns
Cells were lysed in 8 M urea, 100 mM NaH2PO4, 300 mM NaCl, 10 mM Tris-HCl pH 8.0. Lysates were then sonicated with a Bioruptor Plus (Diagenode) for 8 cycles (30 s ON/30 s OFF, at 4 °C) and incubated with Ni-NTA agarose beads (Qiagen) for 1–2 h at room temperature. Beads were washed five times with 8 M urea, 100 mM NaH2PO4, 300 mM NaCl, 10 mM Tris-HCl pH 8.0. Samples were then boiled in loading buffer and subjected to SDS–PAGE and immunoblotting as described above.
Isolation of proteins on nascent DNA
iPOND assays were performed as described59. In brief, 3× 150-mm dishes per sample were labelled with 10 μM EdU for 15 min at 37 °C. After this, cells were immediately fixed by adding 1% paraformaldehyde in PBS to cells after decanting the culture supernatant. After 20 min, cross-linking was quenched with 1 ml of 1.25 M glycine and cells were collected by scraping and transferred to a 50-ml tube. Cells were washed and permeabilized with 0.25% Triton X-100 in PBS for 30 min at room temperature. Click reactions were performed with 10 mM sodium ascorbate, 2 mM CuSO4 and 10 μM biotin azide for 2 h at room temperature. After washing, biotin-labelled cells were lysed (50 mM Tris-HCl pH 8.0, 1% SDS) and sonicated. The cell lysates were incubated with streptavidin magnetic beads overnight at 4 °C. Beads were washed with once with lysis buffer, once with 1 M NaCl, and then twice with lysis buffer. Washed beads were incubated with 2× Laemmli buffer (1:1 (v:v) with beads) and were analysed by SDS–PAGE and immunoblotting.
In vitro binding assay
Flag-tagged KCTD10 protein was generated using the TNT system with wild-type KCTD10 per the manufacturers protocol (Promega). To generate glutathione S-transferase (GST)-tagged wild-type and R167A-mutant KCTD10, the full-length protein was cloned into the pGEX-4T-2 and purified from Escherichia coli using GST Resin (Sigma). The purification was verified by SDS–PAGE as shown in Extended Data Fig. 7l. Purified Flag–KCTD10 and GST–KCTD10 were then incubated together at a 1:1 ratio in ice-cold buffer (20 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, supplemented with 50 mM β-glycerophosphate, 10 mM NaF and protease inhibitors) at 4 °C for 4 h. Following this, GST–KCTD10 was immunoprecipitated and subsequently immunoblotted with an anti-Flag antibody.
Immunofluorescence
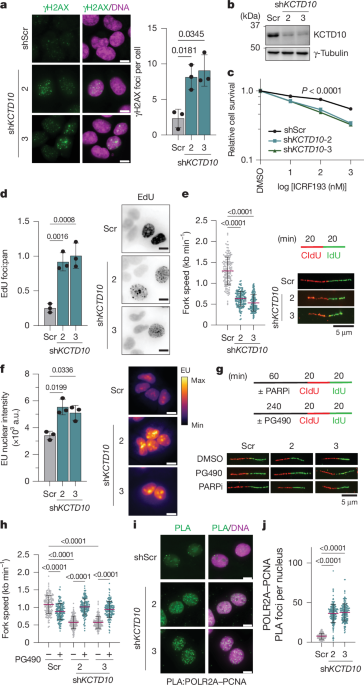
Cells were grown on coverslips for 18–24 h before experiments and then treated as indicated for each experiment. As described previously60, nuclei for RAD51 foci were pre-extracted with (20 mM HEPES pH 7.4, 20 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 1 mM dithiothreitol and protease inhibitor cocktail) for 3 min on ice and fixed in 4% paraformaldehyde for 10 min on ice. After fixation, coverslips were permeabilized with 0.25% Triton X-100 for 10 min at room temperature. Cells were blocked in 3% BSA in PBS for 30 min. Primary and secondary antibodies were diluted in 3% BSA in PBS and incubated on coverslips for 1 h at room temperature. Nuclei were counterstained with Hoechst 33342. Coverslips were mounted onto glass slides using a glycerol-based mounting medium with p-phenylenediamine (10 mg ml−1; Sigma). Coverslips were sealed and imaged on an ImageXpress Micro Confocal system (Molecular Devices).
Nascent DNA imaging
Cells, grown on coverslips, were labelled with EdU (10 μM) for 30 min. Following this, cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 20 min at room temperature. After permeabilization, cells were washed twice in 3% BSA in PBS. Click reactions were then performed with 10 mM sodium ascorbate, 2 mM CuSO4 and 10 μM Alexa Fluor 488 azide for 30 min at room temperature, protected from light. For co-staining proteins, primary and secondary antibodies were added at this point in the same manner described for general immunofluorescence. Nuclei were counterstained with Hoechst 33342, mounted on coverslips and imaged as described above.
Nascent transcription imaging
Nascent transcription imaging was performed using an RNA synthesis kit (abcam). Cells were grown on coverslips and labelled with EU for 1 h. Fixation, permeabilization and click reactions were performed according to the manufacturer’s instructions. Nuclei were counterstained with DAPI as detailed in the protocol. Coverslips were imaged on an ImageXpress Micro Confocal system (Molecular Devices). Nuclear EU intensity was measured in ImageJ using the DAPI channel to create image masks of the nuclei. Intensities for each nuclei are plotted as a single point. For representative images, the mpl-inferno lookup table was applied to the EU channel images.
In situ PLA
Cells were grown on coverslips for 18–24 h before experiments. For PLA assays using 293T cells, cells were grown on poly-l-lysine-coated coverslips to enhance attachment. Cells were pre-extracted for 3 min on ice with nuclear extraction buffer60 (20 mM HEPES, pH 7.4, 20 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 1 mM dithiothreitol and protease inhibitor cocktail). Following this, cells were fixed with 4% paraformaldehyde for 10 min on ice and permeabilized with 0.2% Triton X-100 for 10 min. PLA was performed by a Duo-link in situ PLA kit (Sigma) according to the manufacturer’s instructions. In brief, samples were blocked in blocking solution at 37 °C for 1 h and incubated with primary antibodies (1:500) at room temperature for 1 h. Then, probes were incubated at room temperature for 1 h. Ligation and hybridization were performed at 37 °C for 30 min and 90 min, respectively. Nuclei were counterstained with Hoechst 33342. Coverslips were mounted onto glass slides using a glycerol-based mounting medium with p-phenylenediamine (10 mg ml−1; Sigma) and visualized with an ImageXpress Micro Confocal system (Molecular Devices).
DNA fibre assays
DNA fibre assays were performed as previously described61. In brief, cells were pulsed with 25 μm CldU for 20 min, washed with PBS (37 °C), and pulsed with 250 μm IdU for 20 min. Cells were washed in PBS, counted and resuspended at 1 × 106 per ml. Approximately 2–3 μl of cells were added to the top of a glass slide and dried for approximately 2 min. Cells were then lysed by adding 15 μl lysis buffer (200 mM Tris-HCl pH 7.4, 50 mM EDTA, 0.5% SDS) directly to the cell droplet. The slides were then tilted at an angle by placing them on one end of a tissue culture plate lid allowing DNA fibres to stretch across the slide. Fibres were fixed in 3:1 methanol:acetic acid for 10 min and then air dried. After washing slides with PBS, the fibres were denatured in 2.5 M HCl overnight at 4 °C. Following this, fibres were blocked in 2% BSA in PBS for 30 min and stained with primary antibodies diluted in 2% BSA in PBS for 2 h at room temperature (mouse anti-BrdU (1:25, BD Biosciences) and rat anti-BrdU (1:1,000, abcam)). After washing three times with PBS, fibres were stained with secondary antibodies for 1 h at room temperature (Rhodamine Red anti-rat (1:500) and Alexa Fluor 488 anti-mouse (1:500)). Coverslips were mounted onto slides and images were acquired on a Nikon eclipse 80i fluorescence microscope with a 63× objective. Fibre lengths were measured in ImageJ.
Cell fractionation
Subcellular fraction was performed as described previously with modifications62. In brief, cells were collected, washed in PBS, and lysed for 20 min on ice (10 mM HEPES, pH 7.4, 10 mM KCl, 0.05% NP-40, supplemented with 50 mM β-glycerophosphate, 10 mM NaF and protease inhibitors). After this, nuclei were pelleted by centrifugation in a microcentrifuge (14,000 rpm at 4 °C for 10 min). The nuclei were washed once with lysis buffer and then incubated in low salt buffer for 15 min on ice (10 mM Tris-HCl pH 7.4, 0.2 mM MgCl2, 1% Triton X-100, supplemented with 50 mM β-glycerophosphate, 10 mM NaF, and protease inhibitors). After centrifugation, the soluble fraction was saved as the soluble nuclear proteins. The pellet was resuspended in NETN buffer and sonicated with a Bioruptor Plus (Diagenode) for 8 cycles (30 s ON/30 s OFF, at 4 °C) and centrifuged at 12,500g for 15 min. Lysates were then diluted into Laemmli buffer and analysed by SDS–PAGE with immunoblotting as described above.
RNA/DNA dot blotting
DNA/RNA hybrids were extracted as described63. In brief, cells were lysed in TE buffer with 0.625% SDS and 62.5 μg ml−1 proteinase K for 3 h at 37 °C. DNA was extracted by phenol-chloroform extraction and ethanol precipitated. Precipitated DNA was spooled and washed with 70% ethanol without centrifugation. After air drying DNA, it was resuspended in TE buffer and sonicated. The concentration of DNA was analysed by (Qubit high-sensitivity dsDNA; Invitrogen). Samples were normalized based off the total amount of double stranded DNA (dsDNA) (100 ng μl−1) and were pre-treated with nucleases (RNase H or RNase T1) if indicated. A 2-μl dot of sample was added to a nylon membrane. After the membranes had dried, the nucleic acids were crosslinked by UV irradiation (120 mJ cm−2). Membranes were hydrated in TBS, blocked in 5% milk in TBS, and incubated either S9.6 or dsDNA antibodies (abcam) overnight at 4 °C. Membranes were then washed, stained with secondary antibodies and imaged.
Clonogenic assays
Cells were seeded in triplicate in each well of a 6-well plate (800–1,000 cells per well). After cells attached (16 h later), cells were treated with indicated drugs and left in medium containing the drug for 10–14 days. Colonies were stained with Giemsa solution (Sigma) and counted. Data were normalized to plating efficiencies.
Cell cycle analysis
Cells were trypsinized, collected and filtered through a cell strainer to achieve a single-cell suspension. Ethanol was then added drop-wise with constant mixing to fix and permeabilize cells. Fixed cells were washed three times with PBS, pelleting cells each time with centrifugation at 900g for 5 min at 4 °C. Fixed cells were then resuspended in PI/RNase solution (Thermo Fisher) at room temperature for 30 min. Stained samples were analysed on an AttuneNxT flow cytometer (Thermo Fisher). Cell cycle analysis was performed using FlowJo.
Screening for KCTD10 interaction partners
FASTA sequences belonging the GO term ‘transcription by RNA polymerase II’ (GO:0006366) were retrieved from the European Bioinformatics Institute (EMBL-EBI) Gene Ontology and GO Annotations database (www.ebi.ac.uk/QuickGO/). Using Linux shell commands, the FASTAs were split, concatenated with the full-length FASTA sequence for KCTD10 (UniprotKB ID: Q9H3F6), and then pooled into batches of 100 files. Each batch was run through ColabFold (v1.5.5)34,64,65 using Tesla T4 GPUs as a first pass, then larger complexes were completed using Tesla A100 GPUs. One protein could not be completed due to limitations of AlphaFold 2 (CHD7). iPTM scores were extracted from individual json files and used to create a heat map with rows clustered using Seaborn and matplotlib (Extended Data Fig. 10c). To prioritize top hits, an iPTM score >0.4 was chosen, corresponding to >1 s.d. above the average iPTM scores for the complex formed between KCTD10 and two copies of PCNA. Protein structures were visualized and images for figures were created using ChimeraX-1.6.1. The PAE generated by AlphaFold 2 was plotted as 2D heat maps using ChimeraX-1.6.1.
CUT&RUN sequencing
CUT&RUN was performed according to published protocols66. In brief, 5 × 105 fresh cells were washed twice with wash buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.5 mM spermidine, 1× protease inhibitor cocktail) at room temperature, and incubated with Concanavalin-coated (ConA) magnetic beads (Bangs Laboratories, BP531) prewashed with binding buffer (20 mM HEPES pH 7.9, 10 mM KCl, 1 mM CaCl2, 1 mM MnCl2) at room temperature for 10 min. Then the samples were incubated with primary antibody (mouse anti-Flag, 1:100) in antibody buffer (wash buffer containing 0.05% Digitonin, 2 mM EDTA) on a rotating platform at 4 °C overnight. The beads were then washed with digitonin-containing buffer (wash buffer, 0.05% digitonin), and resuspended with pA/G-MNase (700 ng ml−1 in digitonin buffer) and rotated for 1 h at 4 °C. After two washes with ice-cold digitonin buffer to remove unbound pA/G-MNase, beads were subjected to digestion in pA/G-MNase digestion buffer (digitonin buffer containing 2 mM CaCl2) on ice for 1 h. Solubilized chromatin was then released using CUT&RUN stop buffer (340 mM NaCl, 20 mM EDTA, 4 mM EGTA, 0.05% digitonin, 50 µg ml−1 glycogen and 100 μg ml−1 RNase A) and incubated at 37 °C for 30 min. CUT&RUN DNA was extracted with phenol-chloroform-isoamyl alcohol (Invitrogen, 15593049) and ethanol precipitation and then dissolved in 12 μl 1 mM Tris-HCl pH 8 with 0.1 mM EDTA. The DNA libraries were prepared by NEBNext Ultra II DNA Library Prep Kit for Illumina (E7645) v.2.
Sequencing analysis
DNA libraries were sequenced on a NovaSeq X Plus sequencer. Reads were mapped to the GRCh38 human reference genome using BWA-MEM with default settings67. Sequencing duplicates were marked and removed using Picard implemented in the Genome Analysis Toolkit (GATK4)68. KCTD10 peaks were called using Macs269. Peaks with false discovery rate (FDR) ≤0.05 were considered significant. Sequencing depth in peaks were normalized as reads per kilobase of transcript per million mapped reads (RPKM) using the bamCoverage function in deepTools. Okazaki sequencing data were retrieved from NCBI BioProject PRJNA30158826. RFD was computed using OKseqHMM70. Co-directional or head-on TRCs were identified as described24,25 using a custom script. Genomic feature enrichment in peaks was classified using ChIPseeker package71. Representative regions showing KCTD10 binding regions with TRCs were visualized in Integrative Genomics Viewer (IGV)72. Gene overlap and transcription factor target enrichment analysis was performed using Metascape73.
Figures and schemas
All individual figure panels were made in GraphPad Prism and assembled into figures using Adobe illustrator. Schema were created in Adobe illustrator.
Statistics and reproducibility
All experiments were repeated at least three times independently unless otherwise stated in the figure legends. No statistical methods were used to determine sample size. Investigators were not blinded to the experiment owing to staffing constraints but data were analysed by multiple investigators, some of whom were blinded to the experiment. Immunofluorescence images were acquired and analysed in a blinded fashion. Samples were randomly allocated into experimental groups before treatment and were performed under the same conditions. For example, plates were randomly assigned to groups for experiments. The statistical tests used and resulting P values are indicated in the figures and corresponding legends. Statistical analyses were performed using GraphPad Prism.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.