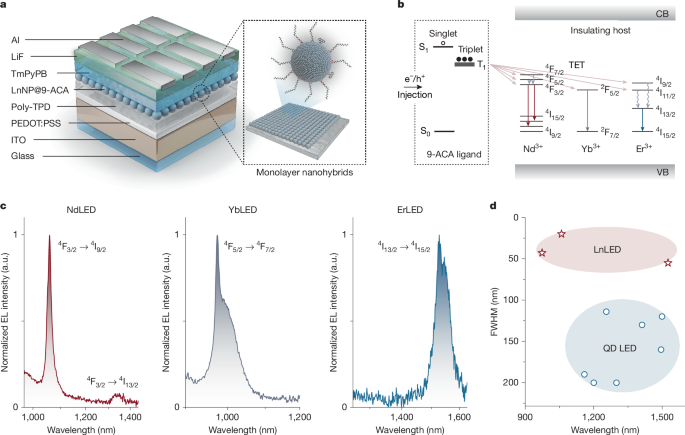
LnNPs consist of an inorganic insulating host, typically fluorides or oxides such as NaGd/Y/LuF4 with a large energy gap of approximately 8 eV (ref. 6), with lanthanide ions embedded in the host lattice. LnNPs have high photo and chemical stability in various environments and have narrow and tunable emission in the NIR-II range (1,000–1,700 nm). This is in contrast to semiconductor-based systems, such as NIR-II emissive organic dyes or semiconducting colloidal quantum dots (QDs), which show broad emission spectra in this region owing to homogeneous broadening. This has motivated research into the application of LnNPs in stimulated-emission depletion microscopy7, deep-tissue theranostics4,8,9,10, sensing11 and optical communication12. However, as these systems are not semiconductors, they cannot be used to construct electrically driven devices, as can be done for colloidal QDs13,14, metal halide perovskites15,16,17 or organic semiconductors18,19.
It has previously been shown that triplet excitons on organic molecules can couple to the f-f transitions in lanthanide ions and that this enables TET between organic molecules and LnNPs20. Organic dye sensitization has proved effective to enhance the emission of LnNPs21,22,23,24,25. Here we use molecular triplet excitons to mediate the function of electrically driven LnNP-based optoelectronic devices, using triplets to efficiently turn on these insulating materials. The first step in this process is to engineer the coupling between organic molecules and LnNPs. The inset of Fig. 1a shows a schematic of the LnNP. The as-prepared LnNPs have oleic acid (OA) on the surface. However, OA is an insulating ligand, which cannot mediate electrical excitation. We therefore partially replace OA with 9-anthracenecarboxylic acid (9-ACA), a widely studied organic dye with a singlet energy of 3.2 eV and triplet energy around 1.8 eV (ref. 26). As shown in Fig. 1b, the triplet energy level of 9-ACA (ref. 27) can, in principle, allow for TET to the ladder-like energy levels of Ln3+ ions (Ln = Nd, Yb, Er). These hybrid materials allow us to construct the first LnLEDs.
a, Schematic illustration of the device architecture of LnLEDs with a close-up schematic of LnNP@9-ACA nanohybrids. b, Simplified schematic showing electron and hole injection through organic molecules to turn on lanthanide ions in an insulating host lattice. CB, conduction band; VB, valence band. c, Normalized EL spectra of LnLEDs. a.u., arbitrary units. d, Reported FWHMs of the EL at different wavelengths from different types of LED, including LnLEDs and QD LEDs.
Figure 1a shows the device architecture of LnLEDs, consisting of glass/indium tin oxide (ITO)/poly(ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS)/poly(4-butylphenyl-diphenylamine) (poly-TPD)/LnNP@9-ACA/1,3,5-tris(3-pyridyl-3-phenyl)benzene (TmPyPB)/lithium fluoride (LiF)/aluminium (Al). ITO and LiF/Al function as electrodes. PEDOT:PSS acts as hole injection layer. TmPyPB and poly-TPD serve as electron and hole transport layers (ETL and HTL), respectively. The LnNP@9-ACA nanohybrids serve as the light-emitting layer. Electrons and holes injected from the contacts travel through the charge transport layers and recombine on the 9-ACA ligands. This will lead to the formation of singlet and triplet excitons on 9-ACA in a 1:3 ratio as governed by the spin–statistics theorem. We note that triplet excitons can undergo efficient energy transfer to the Ln3+ ions as shown in Fig. 1b and as experimentally demonstrated in Fig. 3. The Ln3+ ions can then emit photons, leading to EL from the device. We keep the device architecture constant but vary the type of Ln3+ ions doped into the LnNPs to achieve a range of EL emission from 1,000 to 1,533 nm.
Figure 1c shows the EL spectra obtained from the LnLEDs. The spectra are narrow and consistent with the main peaks of NIR-II photoluminescence (PL) spectra of LnNP@9-ACA nanohybrids under 350-nm photoexcitation. The full widths at half maximum (FWHMs) of LnLEDs EL spectra are calculated to be 20, 43 and 55 nm for Nd/Yb/ErLEDs, which are much lower than the FWHMs found in semiconducting QDs/bulk materials-based systems (FWHM normally above 150 nm)5 (Fig. 1d). The large FWHM of QD LEDs, which is limited by homogeneous line broadening, creates complications for their use in optical communication and chemical/biomedical imaging/sensing applications. The narrow linewidths we achieve here, combined with the inherent ease of processing, flexibility, wide-area compatibility and potential low cost of organic–LnNP hybrids offers exciting possibilities for a new generation of light sources across the NIR-II range. A quantitative comparison of our LnLEDs and other NIR-II LEDs and laser diodes is included in Supplementary Tables 1 and 2.
To obtain high-quality NIR-II light-emitting layers, we synthesized uniform and ultrasmall (<10 nm) LnNPs. Transmission electron microscopy (TEM) images show that all of the LnNPs had good size monodispersity, with an average size of around 6 nm (Supplementary Fig. 1). The dopant ratio of fluorescent Ln3+ (Ln = Nd, Yb, Er) ions has been fixed to 20 mol% in the form of NaGd0.8F4:Ln0.2, which will be subsequently referred to as NdNPs, YbNPs and ErNPs, respectively. This dopant ratio guarantees that enough fluorescent Ln3+ ions receive energy transferred from organic molecules and a fair comparison of energy transfer efficiencies among different Ln3+ ions, while avoiding severe cross-relaxation to maintain a relatively high NIR-II fluorescence28. The high-resolution TEM images and X-ray diffraction (XRD) patterns show that these LnNPs are hexagonal phase (Supplementary Figs. 2 and 3).
As shown in Fig. 2a, the LnNPs show weak and narrow absorption peaks, which is one of the key limitations of LnNPs for various applications. Coupling 9-ACA onto the surface of LnNPs endows LnNP@9-ACA nanohybrids with strong absorption in the ultraviolet range (Fig. 2b). The absorption of these nanohybrids is hence dominated by organic molecules and overcomes the aforementioned limitation of LnNPs. LnNP@9-ACA nanohybrids also show a 5-nm redshift of absorption compared with pure 9-ACA owing to the coupling between organic molecules and LnNPs (Supplementary Fig. 4). Investigations of the ligand exchange process using Fourier-transform infrared (FTIR) spectroscopy and corresponding density functional theory (DFT) simulations (see Fig. 2c,d and Supplementary Figs. 5–7 for details) indicate that the 9-ACA preferentially binds to the Ln3+ ion site on the surface of the LnNPs, in contrast to the OA, which also binds to the Na+ sites. DFT-predicted FTIR spectra of 9-ACA bonded to Gd3+ reproduces the experimentally observed spectrum, whereas 9-ACA bonded to Na+ does not, and introduces peaks at 1,600 cm−1, which are not observed (vertical lines in Fig. 2c). DFT-predicted FTIR spectra for OA show peaks shared at 1,450 and 1,590 cm−1 for OA bonded to Na+ or Gd3+ (vertical lines in Fig. 2d). On the basis of the FTIR data, we estimate the replacement ratios of 9-ACA on different LnNPs to be 6.8%, 1.0% and 3.6% for NdNPs, YbNPs and ErNPs, respectively (Extended Data Fig. 1 and Supplementary Table 3). The important point here is the preferential binding of 9-ACA to the Ln3+ ion sites, which will promote efficient energy transfer.

a,b, Absorption spectra of LnNPs (a) and LnNP@9-ACA nanohybrids (b). c, DFT-simulated FTIR spectra of free 9-ACA molecules, bound 9-ACA molecules to Gd3+ and Na+ ions and experimental data of YbNP@9-ACA nanohybrids. d, DFT-simulated FTIR spectra of free OA molecules, bound OA molecules to Gd3+ and Na+ ions and experimental data of OA-capped YbNPs. e, Comparison of NIR-II emission between LnNPs and LnNP@9-ACA nanohybrids under the excitation of a 350-nm lamp (concentration 20 mg ml−1).
Ligand exchange is a dynamic process and can be influenced by numerous factors. We find that the ligand exchange rate is first increased by prolonging the reaction time and then reaches a plateau, by monitoring the absorbance change of YbNP@9-ACA nanohybrids and PL excitation spectra (Supplementary Fig. 8). We observe that the energy transfer efficiency is not greatly influenced by the ligand exchange rate when the ligand exchange process reaches an equilibrium (Supplementary Figs. 8d–f and 9). We also note that the short distance between 9-ACA and the surface of the LnNPs, linked by the carboxyl group, should allow for efficient TET, as this process is considered to be a Dexter-type energy transfer process. As well as the TET, energy transfer from the singlet state of the 9-ACA to the Ln3+ ions is also possible by means of Förster resonance energy transfer (FRET), although the low absorption cross-section of the Ln3+ ions and poor spectral overlap with the 9-ACA blue emission make this process inefficient29.
As shown in Fig. 2e, the coupling of organic molecules leads to a notable enhancement of the NIR-II emission under ultraviolet excitation, achieving a large Stokes shift. The LnNP@9-ACA nanohybrids show 6.6-fold, 34.1-fold and 23.6-fold enhancement in NIR-II PL compared with NdNPs, YbNPs and ErNPs, respectively. The NIR photoluminescence quantum efficiencies (PLQEs) of LnNPs and LnNP@9-ACA nanohybrids are measured in Supplementary Table 4. Tuning the doping ratio of Ln3+ is a straightforward and effective approach to enhance the fluorescent performance of LnNPs. Increasing the doping ratio of Yb3+ would substantially enhance the downconversion intensities for both YbNPs and YbNP@9-ACA nanohybrids (Supplementary Fig. 10), for which cross-relaxation between Yb3+ ions is not a notable loss, unlike in Er3+ and Nd3+. The ratios of several peaks in the NIR-II EL have changed compared with the PL spectra, indicating distinct energy transfer mechanisms under photoexcitation and electroexcitation for LnNP@9-ACA nanohybrids. To study the energy transfer mechanisms, we further perform steady-state PL, PL decay and transient absorption measurements. Owing to the different amounts of attached 9-ACA in the nanohybrids, we cannot directly compare the intensity of the visible PL to determine the efficiency of the energy transfer (Fig. 3a). PLQE measurements show that bound 9-ACA molecules on LnNPs have markedly decreased PLQE compared with pristine 9-ACA (Supplementary Fig. 11).
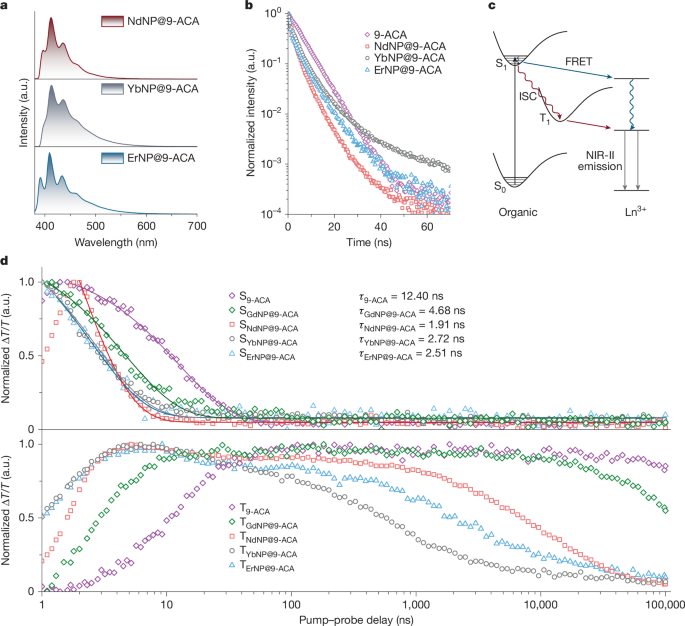
a, Visible emission spectra of LnNP@9-ACA nanohybrids. b, TCSPC measurements of pristine 9-ACA and LnNP@9-ACA nanohybrids under 405-nm laser excitation. c, Schematic demonstration of the accelerated triplet generation by fast ISC when coupling Ln3+ ions and their corresponding energy transfer pathways involving both FRET and TET from organic molecules to Ln3+ ions. d, Kinetics of singlet decay, triplet growth and decay in pristine 9-ACA molecules and molecules attached to different types of LnNPs.
Figure 3b shows time-correlated single photon counting (TCSPC) results for Nd/Yb/ErNP@9-ACA nanohybrids. The lifetime of emission from 9-ACA decreased from 7.97 ns for the pristine ligand to 3.14, 6.77 and 6.94 ns for 9-ACA molecules bound onto Nd/Yb/ErNPs, respectively. Owing to the inevitable presence of free 9-ACA molecules in the nanohybrid solution samples, TCSPC results can merely indicate that energy transfer occurs within the nanohybrids but cannot provide a quantitative analysis of energy transfer efficiency. Hence, pump–probe spectroscopy is applied to reveal the dynamics of photoexcitation and energy transfer between 9-ACA and LnNPs. As shown in Fig. 3d, the S1 states of 9-ACA on NdNP, YbNP and ErNP decay with time constants of 1.91, 2.72 and 2.51 ns, respectively, which is much shorter than the singlet of the pristine 9-ACA and GdNP@9-ACA nanohybrids, which show decay time constants of 12.40 and 4.68 ns, respectively. The rise time constants of the triplet excitons (T1) are measured to be 1.93, 1.41 and 1.39 ns for Nd/Yb/ErNP@9-ACA nanohybrids, respectively. By contrast, the pristine 9-ACA presents a triplet rise time of more than 12.97 ns (Extended Data Fig. 2). This indicates that the singlets of 9-ACA coupled onto LnNPs all undergo rapid intersystem crossing (ISC) and LnNPs increase the rate of the ISC (Fig. 3c), consistent with previous results on the coupling of triplet excitons to the unpaired spin of the doped lanthanide ions20.
Figure 3d also shows much faster decays of the T1 state in Nd/Yb/ErNP@9-ACA nanohybrids compared with the T1 state decay of pristine 9-ACA and GdNP@9-ACA nanohybrids (see also Supplementary Fig. 12). These faster decays are caused by the energy transfer from 9-ACA to the 2F5/2 level of YbNPs, 4F3/2 level of NdNPs and 4I11/2 levels of ErNPs, as GdNPs do not have energy levels available for energy transfer (Fig. 1b). The efficiencies of TET are calculated to be 98.8%, 99.8% and 99.4% for Nd/Yb/ErNP@9-ACA nanohybrids, respectively, based on the quenching of the triplet lifetime compared with GdNP@9-ACA (Extended Data Fig. 2). Both the efficient TET and the less efficient singlet FRET will contribute to the NIR emission of different LnNPs under light excitation. We measured the NIR PL intensities of YbNP@9-ACA nanohybrids under the O2-free and air exposure conditions (Supplementary Fig. 13). The NIR PL in air was quenched by 53.3%, which is consistent with O2 quenching the triplet state of 9-ACA. This further suggests that energy transfer from 9-ACA to LnNPs is mainly mediated by TET. We note three important points about the TET to LnNPs. First, the lifetime of the triplet exciton on 9-ACA is longer than 300 µs, whereas the TET times are on the order of several microseconds. This means that the triplets on the ligands provide a long-lived state from which energy transfer can occur, with few competing kinetic processes, enabling highly efficient transfer (>98%). Second, in Dexter energy transfer, which is the mechanism for TET to LnNPs, the spectral overlap between the triplet phosphorescence spectrum and acceptor absorption spectrum is an important factor20. The phosphorescence spectrum of 9-ACA, which has been reported previously, is broad (roughly 1.3–1.9 eV)27, overlapping with numerous levels within the Ln3+ and allowing for TET. Third, although singlet transfer from the S1 state of 9-ACA to the Ln3+ levels is also possible, the short lifetime of the pristine singlet (12.4 ns), which is further reduced by ISC when attached to the Ln3+ ions with unpaired spins (<5 ns), lowers the efficiency of this pathway, especially given the poor oscillator strength of the Ln3+ ions. The TET pathway is thus the dominant energy transfer pathway and key to enabling the fabrication of optoelectronic devices.
We now turn to the structural characterization of LnLEDs. A schematic of the device structure with flat-band energy levels is shown in Fig. 4a. The device structure is designed to enable charge injection into 9-ACA, leading to exciton formation and subsequent energy transfer to the LnNP. The high-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM) image of the cross-section of the YbLEDs shows the multilayer structure of the hybrid device. The corresponding elemental mapping of the cross-section of the YbLEDs demonstrates a uniform distribution of Yb and Gd elements within the light-emitting layer (Fig. 4b and Supplementary Fig. 14), which is consistent with the scanning electron microscope results of LnNP@9-ACA nanohybrids on PEDOT:PSS/ITO substrates spin-coated at different rotation speeds (Supplementary Fig. 15). Grazing-incidence wide-angle X-ray scattering (GIWAXS) measurements confirm that LnNP@OA and LnNP@9-ACA films on poly-TPD/PEDOT:PSS/ITO do not form a superlattice (Supplementary Fig. 16), with either OA or 9-ACA ligands. The thicknesses of the layers are 25 nm (ITO), 20 nm (PEDOT:PSS), 80 nm (poly-TPD), 15 nm (YbNP@9-ACA), 80 nm (TmPyPB) and 100 nm (LiF/Al), respectively (Fig. 4b). These thicknesses are chosen to enable a relatively high light-extraction efficiency from the emitting layer of the LnLEDs, in the NIR spectral region, as shown in Fig. 4c (see Extended Data Fig. 3 for details).

a, Energy band diagram of LnLEDs. b, Cross-sectional HAADF STEM image of the YbLED and corresponding element mapping of different layers. Scale bars, 50 nm. c, Simulated light-extraction efficiency of the LnLED as a function of emitting wavelength in the NIR range. d–f, Current density and NIR radiance (measured with corresponding long-pass filters) versus voltage for different LnLEDs. g, NIR EQEs of the NdLED/YbLED/ErLED versus current densities. h, Normalized EL spectrum of the Yb@NdLED. i, NIR EQE enhancement by using core–shell Yb@Nd LnNPs and optimizing the device structure.
The NIR-II EL spectra of Nd/Yb/ErLEDs show sharp peaks centred at 1,058, 976 and 1,533 nm, respectively. No shifts in peak emission wavelength were observed under varying driving voltages (Supplementary Fig. 17). Owing to the ladder-like energy levels of LnNPs, there are several peaks for Nd/ErLEDs. The EL of the presented LnLEDs also involves visible-range emission (Supplementary Fig. 18). We assign the blue EL to poly-TPD HTL. The red emission arises from the interface of directly contacted HTL and ETL in the voids of the monolayer LnNP@9-ACA nanohybrids30. No clear EL from 9-ACA is observed, confirming the efficient ISC and TET in the LnNP@9-ACA nanohybrids. The visible EL features suggest electron leakage from ETL or LnNP@9-ACA to poly-TPD, which acts as an efficiency loss channel for the EQEs of LnLEDs.
The current density–voltage–radiance curves of these LnLEDs are shown in Fig. 4g–i. The turn-on voltages for LnLEDs, defined by the voltage corresponding to the minimum measurable radiance in our set-up (0.01 mW sr−1 m−2; see Supplementary Fig. 19), are all around 5 V. The LnLEDs can endure high voltages up to 15 V. As most of the triplets in organic molecules have been transferred to the robust LnNPs, the triplet-induced degradation in LnLEDs could be suppressed. This could allow the LnLEDs to function under high voltages. The peak radiances of the Nd/Yb/ErLEDs are 1.2, 1.2 and 0.4 mW sr−1 m−2, respectively (Fig. 4d–f). The peak EQEs of the Nd/Yb/ErLEDs in the NIR regime are around 0.01%, 0.04% and 0.004%, respectively (Fig. 4g).
The moderate EQEs are limited by the PLQE of the highly doped core-only LnNPs, charge leakage across the emitting layer (indicated by Supplementary Fig. 18) and the decreased light-extraction efficiency in the NIR-II range (Fig. 4c). To further boost the NIR EQEs of LnLEDs, we fabricated core–shell Yb@Nd LnNPs in the form of NaGd0.8F4:Yb0.2@NaGd0.4F4:Nd0.6 to substantially increase the PLQE of Yb@Nd@9-ACA nanohybrids to 3% under 375-nm excitation (Extended Data Fig. 4). Figure 4i compares the NIR EQEs of the Yb@NdLEDs that use this core–shell configuration, together with further optimized HTL with better hole-injection and electron-blocking properties14 and a light out-coupling half-ball lens on substrate. These strategies can suppress the efficiency losses in LnLEDs, eventually boosting the peak NIR EQE of Yb@NdLEDs to greater than 0.6%. We note that the peak EQEs of the LnLEDs are higher than most organic LEDs (OLEDs) emitting above 1,000 nm (ref. 31) (Supplementary Table 1). The EL spectra show a higher Nd/Yb peak intensity ratio of 0.29 than the PL spectra of 0.22 by the direct excitation of Yb@Nd NPs (Fig. 4h and Extended Data Fig. 4), indicating that electrical excitation prefers active surface Ln3+ ions and the energy transfer from Nd3+ to Yb3+ is less efficient than that under light excitation.
We fabricate control LEDs using 9-ACA and NdNP@OA as the emissive layers, respectively, using the same solution-processed method (Supplementary Fig. 20). No emission is detected from the OA-capped NdLEDs. We measure a peak EQE of 0.4% for the pure 9-ACA (without host matrix)-based OLED emitting in the visible range (Supplementary Fig. 20c). This indicates a relatively low electrical-excitation efficiency of the 9-ACA molecule, which could be caused by leakage of charge carriers or interfacial quenching of singlet excitons. These control experiments prove that the molecular antennas are crucial to turn on insulating LnNPs under low voltages.
In summary, this work establishes triplet-mediated electrical excitation as a method to turn on insulating lanthanide nanomaterials, by harvesting the energy from ‘dark’ molecular triplet excitons at low voltages. Using this, we have given the first proof-of-concept demonstration of LnLEDs. These LnLEDs represent the spectrally narrowest NIR-II EL reported so far, with a tolerance for driving voltages of more than 15 V. Our results reveal some of the key energy loss channels that limit this new class of LnLEDs at present, especially the use of monolayers of the nanohybrids and relatively low replacement ratio of 9-ACA on the surface of LnNPs (<10%). This limits the brightness of LnLEDs to a value much lower than that of QD LEDs. But the results also point to strategies to improve these devices in the future. For instance, materials and device optimization will allow for the development of better organic ligands and hosts matrices with optimized charge transport and recombination properties. This effort will be aided by experience built up in the OLED community over several decades. On the emitter side, we note that the PLQEs of ultrasmall LnNPs with high dopant concentration used in this study are very modest. These modest PLQEs serve as another loss channel and cap the efficiency of these first devices (EQE >0.6%). In the future, tuning the doping ratio and doping types of Ln3+, as well as control of particle size, will boost the NIR-II PLQEs32,33, thus allowing for higher EQEs. For instance, it has been shown that PLQEs >50% can be achieved for Er3+ emission at 1,530 nm in LnNPs32. Future experimental and theoretical work will thus be required to enhance the brightness and operational stability of LnLEDs (Extended Data Fig. 4). The general method we establish here can be applied to numerous organic molecules and various LnNPs, providing a new route to turn on insulating materials under low applied biases. This opens a new field for the design and fabrication of hybrid LEDs and also other electrically pumped devices, such as lasers, with huge potential in biomedical theranostics, optogenetics and optical communication.