Update: After this story was published, an updated draft agenda for the ACIP meeting was posted. According to the schedule, the committee will no longer consider the RSV vaccine, but it will vote on the three other shots described in this story: COVID-19, hepatitis B and MMRV.
Next week, the panel of top advisers who recommend how vaccines are used in the United States will meet to review jabs that protect against COVID-19, hepatitis B and other diseases. The meeting — the committee’s second since US health secretary and anti-vaccine advocate Robert F. Kennedy Jr abruptly fired all of its previous 17 members and welcomed 7 new ones — has raised eyebrows among public-health specialists, given that the safety and efficacy of some of the vaccines on the agenda have been well established for years.
“I’m very concerned, given the signalling from the members of this newly reconstituted, hand-picked [committee] that they are going to select targets for further restriction,” says Andrew Pavia, a physician with the Infectious Diseases Society of America, a medical association based in Arlington, Virginia. At their last meeting, the advisers — several of whom have publicly expressed anti-vaccine views — voted to end the use of the preservative thimerosal in influenza vaccines, despite evidence that it is safe at the doses found in jabs.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
It is unclear exactly what the Advisory Committee on Immunization Practices (ACIP) will vote on at next week’s meeting, to be held on 18 and 19 September, because the agenda lacks detail, increasing researchers’ concern. “It’s very unusual for ACIP to put things on the agenda with only a month’s notice and not discuss who’s going to be presenting data, what data is going to be looked at, and what, if any, questions might come up for a vote,” Pavia says.
The US Department of Health and Human Services, which is run by Kennedy and has authority over the ACIP, did not respond to Nature’s request for comment.
Nature spoke to public-health specialists about the vaccines up for discussion, what the committee might vote on and the data it should be considering.
Scrutiny for COVID-19 jabs
Last month, the US Food and Drug Administration (FDA) approved updated COVID-19 vaccines, but it imposed limitations on who can get the shots. Whereas the vaccines were previously authorized for all people aged 6 months and older in the United States, now they are approved only for those older than 65 years, as well as people with health conditions that put them at high risk of severe disease.
The FDA is responsible for authorizing new vaccines, whereas the ACIP, overseen by the US Centers for Disease Control and Prevention (CDC), advises on who should receive them. These recommendations, which are typically adopted by the CDC as official policy, help to inform which vaccines are covered by health insurance.
“I assume ACIP could end up mirroring what the FDA has recommended” for COVID-19 shots, says Adam Ratner, a paediatrician at the American Academy of Pediatrics (AAP), an organization based in Itasca, Illinois.
In a statement following the FDA’s decision on 27 August, AAP president Susan Kressly called the move “deeply troubling”, especially for children, whose safety Kennedy has focused on when questioning vaccines. The previous week, the AAP issued its own recommendation that all children 6 months and older should be vaccinated and, in particular, kids aged 6–23 months old who are at high risk of severe COVID-19.
According to data from the 2022–23 cold and flu season, mRNA-based COVID-19 vaccines were between 46% and 70% effective at preventing COVID-19-related emergencies for children aged 6 months up to 5 years during a 2-month window after the second or third dose. (Flu vaccines, which are also updated seasonally, are typically 40–60% effective.) And such shots elicited no safety concerns in that same age group.
However, The Washington Post is reporting that Trump health officials might link COVID-19 vaccines with 25 paediatric deaths reported to a vaccine safety database at next week’s meeting. Anyone can submit reports to the database, but claims must be investigated to confirm a link.
Hepatitis B shots no longer for newborns?
Anti-vaccine advocates have been questioning whether a hepatitis B vaccine should be given to newborns — the current practice in the United States. Instead, they are suggesting that jabs be administered only to people with certain high-risk behaviours, including unprotected sex and drug use, Pavia says. That’s a problem, he adds, because “the majority of hepatitis B worldwide is transmitted from mother to infant”.
Hepatitis B is a liver disease caused by a virus that spreads through contact with infected blood and other body fluids. According to the AAP, babies infected with hepatitis B in their first year of life have a 90% chance of developing chronic disease, and one-quarter of those with chronic hepatitis B die from it.
Nature; Source: CDC/National Notifiable Diseases Surveillance System (data)
Since the United States began recommending hepatitis B vaccines for infants in the 1990s, cases have dropped significantly (see ‘Generational decline in hepatitis B’). Administering “earlier is always better with an incurable virus”, says Peter Chin-Hong, an infectious-disease physician at the University of California, San Francisco.
Some think that the ACIP panel will recommend doing away with hepatitis B vaccines for infants, instead relying on screening pregnant people for the disease — and vaccinating only the infants of those who test positive. But “there can be false negatives”, Ratner says. And “there can be people who are screened, and then the result is not communicated efficiently” so action is not taken. Giving the vaccine at birth is therefore the most robust protection, he adds. Studies have demonstrated the safety of vaccinating newborns for hepatitis B.
Stricter advice for measles jabs?
In 2008, the US vaccine safety system identified an increased risk of fever-induced seizures in children between one and two years of age who received the measles, mumps, rubella and varicella (MMRV) vaccine (varicella is commonly known as chickenpox). That observation led the CDC to recommend that children in that age group receive separate shots for their first dose — one for measles, mumps and rubella (MMR) followed by one for varicella — unless a parent expressed a preference for the single MMRV shot. For the second dose, typically given to children 4–6 years of age, the agency recommended the combination MMRV jab, because there weren’t safety concerns for that age group.
During the last ACIP meeting, chair Martin Kulldorff presented the data from 2008 and proposed that the MMRV vaccine never be administered to children under the age of four years. This is in line with what the CDC already recommends, so it’s possible that Kulldorff simply wants to strengthen the language of the guidance. Still, strongly opposing the MMRV jab could have its downsides: the combination shot makes it easier for people to comply with vaccination recommendations because it requires fewer visits to a clinic, Chin-Hong says.
RSV shots for pregnant people
The respiratory syncytial virus (RSV) is a major cause of hospitalization among babies in the United States. Two ways exist for protecting infants from the disease, which inflames the smallest airways of the lungs. Babies can receive a monoclonal antibody during their first eight months, or a pregnant person can get an RSV vaccine, which in turn protects their infant (see ‘Born with RSV protection’).
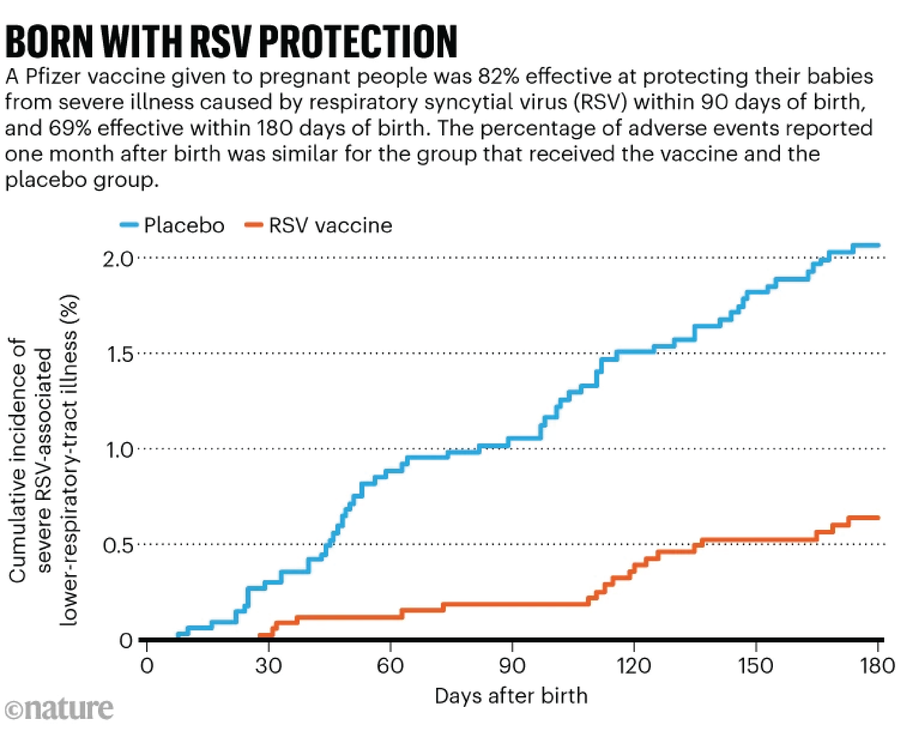
During the last ACIP meeting, committee members voted to recommend a monoclonal antibody manufactured by pharmaceutical company Merck for babies under eight months not protected from RSV by vaccination during gestation. It’s not clear what the committee will be discussing regarding RSV this time, but one possible target is vaccination of pregnant people. Currently, the CDC recommends that they receive one dose of an RSV vaccine between 32 and 36 weeks of gestation. In a clinical trial of the shot, which is manufactured by pharmaceutical company Pfizer, there were slightly more preterm births in the vaccine group than in the placebo group, but the increase was not statistically significant.
“If we had a perfect system where every child got the monoclonal antibody preparation, you might not need maternal vaccinations,” Ratner says. “But in the real world, that doesn’t always happen and there may be barriers” for babies to access the preventive treatment, such as the hospital failing to offer it after birth.
This article is reproduced with permission and was first published on September 12, 2025.

