Materials and instruments
All reagents and solvents used for the synthesis of the compounds were purchased from Aldrich and Acros companies and used without further purification. 1H nuclear magnetic resonance (NMR) spectra were recorded using a Varian Mercury plus 400NB spectrometer, with tetramethylsilane (TMS) as the internal standard. Molecular masses were determined using a FINNIGAN LCQ electrospray ionization mass spectrometer or a matrix-assisted laser desorption/ionization time-of-flight mass spectrometer. Elemental analyses were performed using a Vario EL III elemental analyser. Suitable single crystals for X-ray diffraction analysis were obtained by slowly diffusing 12 ml of n-hexane into a 3-ml dichloromethane solution of ArPPOA (10 mg) at room temperature. X-ray diffraction data were collected at 295 K on a Rigaku Xcalibur E diffractometer with graphite-monochromatized Mo Kα radiation (λ = 0.71073 Å) in ω scan mode. The structures were solved using direct methods and difference Fourier syntheses. Non-hydrogen atoms were refined by full-matrix least-squares techniques on F2 with anisotropic thermal parameters. Hydrogen atoms attached to carbons were placed at calculated positions (C–H = 0.93 Å) with U(H) = 1.2Ueq(C), following the riding model approximation. All calculations were carried out using the SHELXL97 program.
We performed transmission electron microscopy measurements using a field-emission transmission electron microscope (JEOL JEM-2010F) operated at an acceleration voltage of 200 kV. Absorption and PL emission spectra were measured using a Shimadzu UV-3150 spectrophotometer and a Shimadzu RF-5301PC spectrophotometer, respectively. Cyclic voltammetry was conducted using an Eco Chemie B.V. Autolab potentiostat in a three-electrode cell with a glassy carbon working electrode, a platinum wire counter electrode and a silver/silver chloride (Ag/AgCl) reference electrode. Electrochemical experiments were carried out under a nitrogen atmosphere at room temperature in dichloromethane. Phosphorescence spectra were measured using an Edinburgh FLS1000 fluorescence spectrophotometer at 50 K.
Time decay spectra were measured using the time-correlated single photon counting method with a picosecond hydrogen lamp for the 100 ps to 10 μs range and a microsecond pulsed xenon light source for 1 μs to 10 s lifetime measurements. The synchronization photomultiplier collected the signal and the multi-channel scaling mode of the PCS900 fast counter PC plug-in card was used for data processing. Prompt and delayed fluorescence lifetimes were respectively measured with nanosecond and microsecond time decay methods. Lifetime values were simulated using an exponential fitting function in Fluoracle software.
Nanocrystal-based films (20–40 nm) for optical analysis were prepared through spin coating. The PLQYs of these films were measured using a Labsphere 1-M-2 integrating sphere (ϕ = 6”) coated by BenFlect, providing efficient light reflection across a wide range of 200–1,600 nm. The integrating sphere was coupled with the FLS1000 system. The absolute PLQY was determined by recording two spectral (emission) scans. The first spectrum captured both the scattered light and the emission from the sample, whereas the second spectrum measured the scattered light from the BenFlect coating. By integrating and subtracting the scattered light parts from both spectra, we determined the photon number absorbed by the sample (Na). The emission of the sample was integrated to calculate the emissive photon number (Ne). The absolute PLQY (η) was then calculated using the equation η = Ne/Na. Spectral correction (emission arm) was applied to the raw data after background subtraction and the quantum yield was calculated from the spectrally corrected curves using the F900 software wizard.
Synthesis details
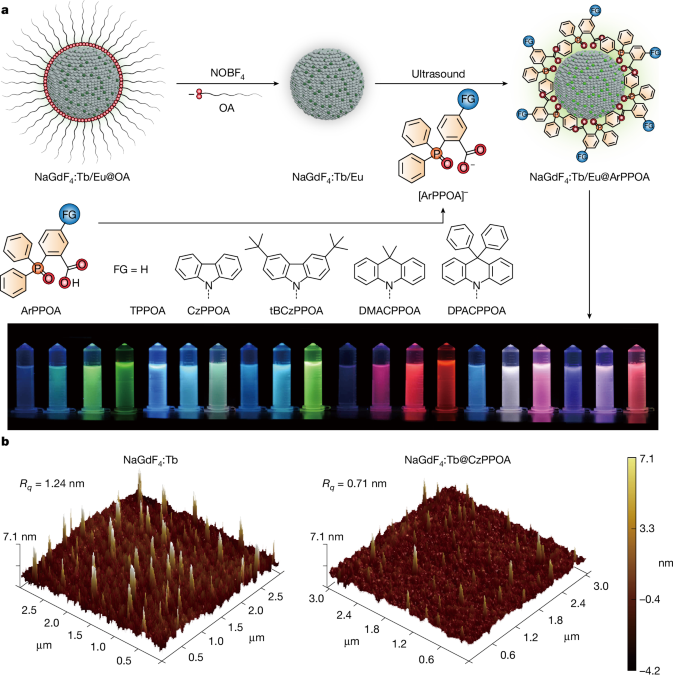
NaGd1-xF4:Tb/Eux@OA nanocrystals: Lanthanide nanocrystals were synthesized according to a well-documented coprecipitation method30. In a typical experiment for synthesizing NaGd1-xF4:Tbx nanocrystals, GdCl3·6H2O (1-x mmol) and TbCl3·6H2O or EuCl3·6H2O (x mmol) were mixed with OA (6 ml) and 1-octadecene (15 ml) in a 100-ml flask. The mixture was heated to 140 °C for 3 h. After cooling to 50 °C, a methanol solution (10 ml) containing NaOH (0.1 g, 2.5 mmol) and NH4F (0.148 g, 4 mmol) was added and the mixed solution was stirred for 12 h. The temperature was then raised to 70 °C to remove methanol. After that, the solution was heated to 240 °C under an argon atmosphere for 45 min, followed by cooling to room temperature. The resulting nanocrystals were extracted by repeated precipitation with a mixture of ethanol and hexane, collected by centrifugation at 12,000 rpm for 5 min and redispersed in 9 ml of hexane.
NaGd0.6F4:Tb0.4−xEu@OA nanocrystals
The synthesis followed the same procedure as above, with the inclusion of EuCl3·6H2O (x mmol, x = 0.01, 0.05, 0.08 or 0.10).
Preparation of ligand-free nanocrystals
In a typical process32, 1 ml of the as-prepared OA-capped nanocrystals dispersion in hexane (about 50 mg ml−1) was combined with 1 ml of a N,N-dimethylformamide (DMF) solution containing NOBF4 (0.011 g, 0.1 mmol) at room temperature. The mixture was ultrasonicated for 20 min to remove oleate ligands on the surface, followed by the addition of 1 ml of toluene and further sonication for another 20 min. The ligand-free nanocrystals were collected by centrifugation and redispersed in DMF (1 ml). For purification, 1 ml of a hexane–toluene solution (1:1 v/v) was added to flocculate the dispersion and the precipitate was collected by means of centrifugation. The nanocrystals were then redispersed in 2 ml of EtOH to form a stable colloidal dispersion.
Surface ligand modification
For ligand modification, sodium hydroxide (0.002 g, 0.05 mmol) in 1 ml of ethanol was added to the desired ligand (0.05 mmol) in 2 ml of ethanol to prepare a ligand salt solution. This solution was added to an ethanol dispersion of ligand-free nanocrystals (0.001 mmol) and ultrasonicated for two hours to ensure ligand coordination to nanocrystal surfaces. Excess ligand was removed by centrifugation and the modified nanocrystals were redispersed in ethanol for optical measurements or in DMF for device fabrication.
Diphenyl(o-tolyl)phosphine oxide (TPPOM)
Under an argon atmosphere, 1-bromo-2-methylbenzene (1.186 ml, 10 mmol) in 10 ml of dry ether was added dropwise to a mixture of magnesium turnings (0.267 g, 11 mmol) and a small piece of iodine in 10 ml of dry ether at room temperature. The reaction was stirred at 40 °C for one hour. After cooling to 0 °C, chlorodiphenylphosphine (1.980 ml, 11 mmol) in 10 ml of dry ether was added dropwise and stirred for 12 h. The reaction was quenched by adding water and the mixture was extracted with CH2Cl2 (3 × 30 ml). The CH2Cl2 solution was concentrated to 30 ml, then 30% H2O2 (4.5 ml, 40 mmol) was added at 0 °C and stirred for four hours. After another extraction with CH2Cl2 (3 × 30 ml), the organic phase was combined and dried with anhydrous Na2SO4. The solvent was removed in vacuo. The product was purified by flash column chromatography, affording 2.6 g of white powder in 90% yield. 1H NMR (TMS, CDCl3, 400 MHz): δ = 7.708–7.612 (m, 4H), 7.591–7.519 (m, 2H), 7.515–7.390 (m, 5H), 7.319–7.270 (m, 1H), 7.115 (t, J = 7.2 Hz, 1H), 6.998 (q, J1 = 13.6 Hz, J2 = 7.2 Hz, 1H), 2.453 ppm (s, 3H). Laser desorption/ionization time-of-flight (LDI-TOF): m/z (%): 292.10 (100) [M+]; elemental analysis (%) for C19H17OP: C 78.07, H 5.86, O 5.47; found: C 78.09, H 5.89, O 5.50.
(4-bromo-2-methylphenyl)diphenylphosphine oxide (TPPOMBr)
The synthetic procedure was similar to that of TPPOM except for using 4-bromo-1-iodo-2-methylbenzene (2.959 g, 10 mmol) instead of 1-bromo-2-methylbenzene. The product yielded 3.3 g of white powder (90% yield). 1H NMR (TMS, DMSO-d6, 400 MHz): δ = 7.680–7.605 (m, 3H), 7.604–7.525 (m, 8H), 7.489 (d, J = 8.0 Hz, 1H), 6.857 (q, J1 = 13.2 Hz, J2 = 8.4 Hz, 1H), 2.288 ppm (s, 3H). LDI-TOF: m/z (%): 370.01 (100) [M+]; elemental analysis (%) for C19H16BrOP: C 61.48, H 4.34, O 4.31; found: C 61.50, H 4.36, O 4.35.
(4-(9H-carbazol-9-yl)-2-methylphenyl)diphenylphosphine oxide (CzPPOM)
Under an argon atmosphere, TPPOMBr (1.856 g, 5 mmol), carbazole (2.508 g, 15 mmol), CuI (0.095 g, 0.5 mmol) and K2CO3 (2.073 g, 15 mmol) were dissolved in 50 ml of 1,3-dimethyl-2-imidazolidinone (DMI) and heated to 190 °C for 12 h. After cooling to room temperature, the mixture was poured into water and extracted with dichloromethane (3 × 10 ml) again. The organic layers were combined and dried with anhydrous Na2SO4 and the solvent was removed in vacuo. The crude product was purified by column chromatography, affording 1.8 g of white powder (80% yield). 1H NMR (TMS, DMSO-d6, 400 MHz): δ = 8.231 (d, J = 7.6 Hz, 2H), 7.753–7.694 (m, 3H), 7.693–7.643 (m, 4H), 7.644–7.577 (m, 4H), 7.542 (d, J = 8.0 Hz, 1H), 7.497 (d, J = 8.0 Hz, 2H), 7.411 (t, J = 7.6 Hz, 2H), 7.279 (t, J = 7.2 Hz, 2H), 7.209 (q, J1 = 13.6 Hz, J2 = 8.4 Hz, 1H), 2.432 ppm (s, 3H). LDI-TOF: m/z (%): 457.16 (100) [M+]; elemental analysis (%) for C31H24NOP: C 81.38, H 5.29, N 3.06, O 3.50; found: C 81.39, H 5.30, N 3.08, O 3.54.
(4-(3,6-di-tert-butyl-9H-carbazol-9-yl)-2-methylphenyl)diphenylphosphine oxide (tBCzPPOM)
The synthetic procedure was similar to that of CzPPOM except for using 3,6-di-tert-butyl-carbazole (4.188 g, 15 mmol) instead of carbazole. The yield was 2.2 g of white powder (80% yield). 1H NMR (TMS, CDCl3, 400 MHz): δ = 8.138 (s, 2H), 7.711 (q, J1 = 11.6 Hz, J2 = 7.6 Hz, 4H), 7.480 (s, 1H), 7.448–7.332 (m, 10H), 7.299 (d, J = 8.0 Hz, 1H), 7.220 (q, J1 = 13.2 Hz, J2 = 8.0 Hz, 1H), 2.515 (s, 3H), 1.385 ppm (s, 18H). LDI-TOF: m/z (%): 569.28 (100) [M+]; elemental analysis (%) for C39H40NOP: C 82.22, H 7.08, N 2.46, O 2.81; found: C 82.25, H 7.07, N 2.50, O 2.83.
(4-(9,9-dimethylacridin-10(9H)-yl)-2-methylphenyl)diphenylphosphine oxide (DMACPPOM)
In an argon atmosphere, tris(dibenzylideneacetone)dipalladium (0.366 g, 0.4 mmol) and (t-Bu)3P (0.094 ml, 0.4 mmol) were mixed in toluene (10 ml) and stirred for 20 min at room temperature. TPPOMBr (3.700 g, 10 mmol), 9,9-dimethyl-9,10-dihydroacridine (DMAC, 2.509 g, 12 mmol), t-BuONa (1.922 g, 20 mmol) were added and the mixture was heated to 90 °C and stirred for six hours. After the reaction, the toluene solvent was removed by distillation and the solid was dissolved in dichloromethane. The crude product was purified by flash column chromatography, yielding 3.9 g of pale-yellow powder (80% yield). 1H NMR (TMS, CDCl3, 400 MHz): δ = 7.739 (q, J1 = 12.0 Hz, J2 = 6.8 Hz, 4H), 7.581 (t, J = 7.2 Hz, 2H), 7.570–7.499 (m, 4H), 7.438 (dd, J1 = 7.6 Hz, J2 = 1.2 Hz, 2H), 7.305–7.267 (m, 1H), 7.268–7.215 (m, 1H), 7.120 (d, J = 8.0 Hz, 1H), 6.978 (t, J = 7.2 Hz, 2H), 6.920 (t, J = 7.2 Hz, 2H), 6.266 (d, J = 8.0 Hz, 2H), 2.519 (s, 3H), 1.667 ppm (s, 6H). LDI-TOF: m/z (%): 499.21 (100) [M+]; elemental analysis (%) for C34H30NOP: C 81.74, H 6.05, N 2.80, O 3.20; found: C 81.75, H 6.06, N 2.84, O 3.22.
(4-(9,9-diphenylacridin-10(9H)-yl)-2-methylphenyl)diphenylphosphine oxide (DPACPPOM)
The synthetic procedure was similar to that of DMACPPOM except for using 9,9-diphenyl-9,10-dihydroacridine (DPAC, 3.998 g, 12 mmol) instead of DMAC. The reaction yielded 4.9 g of white powder with an 80% yield. 1H NMR (TMS, CDCl3, 400 MHz): δ = 7.689 (q, J1 = 12.0 Hz, J2 = 7.2 Hz, 4H), 7.567 (t, J = 7.2 Hz, 2H), 7.550–7.464 (m, 4H), 7.291–7.193 (m, 6H), 7.126 (q, J1 = 13.6 Hz, J2 = 8.0 Hz, 1H), 7.112–7.039 (m, 2H), 7.004–6.927 (m, 5H), 6.922–6.843 (m, 5H), 6.425 (d, J = 8.0 Hz, 2H), 2.444 ppm (s, 3H). LDI-TOF: m/z (%): 623.24 (100) [M+]; elemental analysis (%) for C44H34NOP: C 84.73, H 5.49, N 2.25, O 2.57; found: C 84.74, H 5.51, N 2.28, O 2.60.
2-(diphenylphosphoryl)benzoic acid (TPPOA)
Powdered KMnO4 (3.161 g, 20 mmol) was added in four portions over 1.5 h to a boiling mixture of diphenyl(o-tolyl)phosphine oxide (TPPOM) (1.461 g, 5 mmol), pyridine (25 ml) and water (10 ml), maintaining gentle boiling throughout. The mixture was boiled for 5 h, after which pyridine and water were removed by distillation. On cooling to room temperature, 1 ml (6 mmol ml−1) of hydrochloric acid in 10 ml of H2O was added dropwise and stirred for 30 min. The mixture was extracted with water and chloroform (3 × 10 ml). The organic layers were combined and dried with anhydrous Na2SO4. The solvent was removed in vacuo. The crude product was purified by column chromatography, yielding 1.1 g of white powder with a yield of 70%. 1H NMR (TMS, DMSO-d6, 400 MHz): δ = 13.086 (s, 1H), 7.904–7.845 (m, 1H), 7.701 (t, J = 7.6 Hz, 1H), 7.628 (t, J = 7.6 Hz, 1H), 7.610–7.539 (m, 5H), 7.539–7.455 ppm (m, 6H). LDI-TOF: m/z (%): 322.08 (100) [M+]; elemental analysis (%) for C19H15O3P: C 70.81, H 4.69, O 14.89; found: C 70.83, H 4.69, O 14.91.
5-(9H-carbazol-9-yl)-2-(diphenylphosphoryl)benzoic acid (CzPPOA)
The synthetic procedure was analogous to that of TPPOA but with the substitution of CzPPOM (2.286 g, 5 mmol) for TPPOM. The reaction produced 0.9 g of white powder with a 40% yield. 1H NMR (TMS, DMSO-d6, 400 MHz): δ = 13.386 (s, 1H), 8.256 (d, J = 7.6 Hz, 2H), 8.090 (t, J = 2.0 Hz, 1H), 7.975 (d, J = 8.4 Hz, 1H), 7.762 (q, J1 = 12.8 Hz, J2 = 8.4 Hz, 1H), 7.738–7.645 (m, 4H), 7.642–7.588 (m, 2H), 7.588-7.510 (m, 6H), 7.447 (t, J = 7.6 Hz, 2H), 7.314 ppm (t, J = 7.6 Hz, 2H). LDI-TOF: m/z (%): 487.13 (100) [M+]; elemental analysis (%) for C31H22NO3P: C 76.38, H 4.55, N 2.87, O 9.85; found: C 76.39, H 4.57, N 2.89, O 9.88.
5-(3,6-di-tert-butyl-9H-carbazol-9-yl)-2-(diphenylphosphoryl)benzoic acid (tBCzPPOA)
The synthetic procedure was similar to that of TPPOA except for using tBCzPPOM (2.846 g, 5 mmol) instead of TPPOM. The reaction yielded 1.2 g of white powder with a 40% yield. 1H NMR (TMS, DMSO-d6, 400 MHz): δ = 13.359 (s, 1H), 8.324 (s, 2H), 8.077 (s, 1H), 7.977 (d, J = 6.8 Hz, 1H), 7.821–7.720 (m, 1H), 7.718–7.631 (m, 4H), 7.629–7.527 (m, 6H), 7.525–7.424 (m, 4H), 1.416 ppm (s, 18H). LDI-TOF: m/z (%): 599.26 (100) [M+]; elemental analysis (%) for C39H38NO3P: C 78.11, H 6.39, N 2.34, O 8.00; found: C 78.13, H 6.37, N 2.37, O 8.03.
5-(9,9-dimethylacridin-10(9H)-yl)-2-(diphenylphosphoryl)benzoic acid (DMACPPOA)
The synthetic procedure was similar to that of TPPOA except that DMACPPOM (2.496 g, 5 mmol) was used instead of TPPOM. The reaction gave 1.0 g of pale-yellow powder with a 40% yield. 1H NMR (TMS, CDCl3, 400 MHz): δ = 8.392 (s, 1H), 7.750–7.548 (m, 9H), 7.545–7.432 (m, 5H), 7.324–7.254 (m, 2H), 7.252–7.175 (m, 2H), 7.128 (t, J = 7.2 Hz, 1H), 7.036 (d, J = 7.6 Hz, 1H), 1.486 ppm (s, 6H). LDI-TOF: m/z (%): 529.18 (100) [M+]; elemental analysis (%) for C34H28NO3P: C 77.11, H 5.33, N 2.64, O 9.06; found: C 77.11, H 5.32, N 2.67, O 9.08.
5-(9,9-diphenylacridin-10(9H)-yl)-2-(diphenylphosphoryl)benzoic acid (DPACPPOA)
The synthetic procedure was similar to that of TPPOA except that DMACPPOM (3.116 g, 5 mmol) was used instead of TPPOM. The reaction yielded 1.3 g of white powder with a 40% yield. 1H NMR (TMS, CDCl3, 400 MHz): δ = 8.097 (s, 1H), 7.652–7.527 (m, 6H), 7.524–7.423 (m, 4H), 7.294–7.165 (m, 6H), 7.106 (t, J = 7.2 Hz, 2H), 7.028 (q, J1 = 14.0 Hz, J2 = 8.0 Hz, 1H), 7.000–6.854 (m, 9H), 6.572 ppm (d, J = 8.0 Hz, 2H). LDI-TOF: m/z (%): 653.21 (100) [M+]; elemental analysis (%) for C44H32NO3P: C 80.84, H 4.93, N 2.14, O 7.34; found: C 80.86, H 4.92, N 2.16, O 7.38.
Nanohybrid synthesis
To prepare the ligand salt solution, sodium hydroxide (0.002 g, 0.05 mmol) in 1 ml of ethanol was added into a mixture of 0.05 mmol of ligand in 2 ml of ethanol. This ligand salt solution was then combined with an ethanol solution containing ligand-free nanocrystals (0.001 mmol) and the mixture was ultrasonicated for two hours to ensure proper ligand coordination of the ligands to nanocrystal surfaces. Any excess ligand was removed by centrifugation and the resulting product was redispersed in ethanol for optical measurements or in DMF for device fabrication.
Preparation of Tb(ligand)3 complexes
Tb(ligand)3 complexes were prepared according to established protocols33. ArPPOA (3 mmol) was dissolved in 10 ml of ethanol and NaOH (0.120 g, 3 mmol) in aqueous solution (1 M) was added to deprotonate ArPPOA. TbCl3·6H2O (0.373 g, 1 mmol) in 0.1 ml of water was added dropwise, then the solution was stirred at 60 °C for two hours. The product was purified by precipitation using a concentrated ethanol–water solution.
Device fabrication
The device structure consisted of: ITO|PEDOT:PSS (40 nm)|PVK (20 nm)|mCP:y wt% NaGd0.6F4:Tb0.4−xEux@ligand (25 nm)|DPEPO (10 nm)|TmPyPB (40 nm)|LiF (1 nm)|Al (100 nm). In this configuration, poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) and LiF serve as the hole and electron injection layers, respectively, whereas the other materials, including polyvinylcarbazole (PVK), 1,3-bis(N-carbazolyl)benzene (mCP), bis[2-(diphenylphosphino)phenyl] ether oxide (DPEPO) and 1,3,5-tri[(3-pyridyl)-phen-3-yl]benzene (TmPyPB), function as hole transporting, host, exciton blocking and electron transporting layers, respectively. Further host materials such as bis-4-(N-carbazolyl)phenyl)phenylphosphine oxide (BCPO), 4,4′-bis(9H-carbazol-9-yl)biphenyl (CBP), 4,4-bis(9-carbazolyl)-2,2-dimethylbiphenyl (CDBP), CPPOM, 9-(4-tert-butylphenyl)-3,6-bis(triphenylsilyl)-9H-carbazole (CzSi), DPEPO and PVK were also used for comparison. The PEDOT:PSS layer was spin-coated on a patterned ITO-coated glass substrate after oxygen plasma treatment. To remove any residual water, the PEDOT:PSS layer was baked at 120 °C for 20 min in a glovebox. The PVK layer was then spin-coated from a 10 mg ml−1 DMF solution onto the PEDOT:PSS layer and baked at 70 °C for 15 min. The emitting layer, also spin-coated from DMF at a concentration of 10 mg ml−1, was similarly baked at 70 °C for 15 min. After spin-coating, the sample was transferred to a high-vacuum evaporation system. The electron transporting layers were sequentially evaporated at a rate of 0.1-0.2 nm s−1 under a pressure less than 4 × 10−4 Pa. A 1-nm-thick LiF layer was deposited at 0.1 nm s−1 to improve electron injection, followed by a 100-nm-thick Al cathode deposited at 0.6 nm s−1. The emission area of the devices was 0.09 cm2, defined by the overlap of the anode and cathode. Post-fabrication, all devices were encapsulated with ultraviolet epoxy resin in the glovebox before undergoing luminance–current–voltage measurements. Emission intensity was measured with a calibrated Si photodiode and the external quantum efficiency was calculated assuming a Lambertian distribution. The electroluminescent spectrum was recorded using a calibrated PR-655 spectrometer.
Absorption and luminescence spectroscopy analysis
Absorption spectra in the near-infrared range were measured at room temperature using a Shimadzu ultraviolet–visible–near-infrared spectrophotometer (UV-3600). PL spectra were recorded at room temperature using a DM150i monochromator and an R928 photon-counting photomultiplier tube, in conjunction with a 980-nm diode laser. Decay curves were measured with a custom ultraviolet-to-mid-infrared phosphorescence lifetime spectrometer (FLS1000, Edinburgh) equipped with a digital oscilloscope (TDS3052B, Tektronix) and a tunable optical parametric oscillator laser (410–2,400 nm, Vibrant 355 II, OPOTEK) as the excitation source.
Transient absorption spectroscopy
Transient absorption spectra were recorded using a pump–probe set-up. Samples were excited by tunable pump pulses (355–2,600 nm) generated from an optical parametric amplifier, pumped by a regenerative Ti:sapphire amplifier (Coherent; 800 nm, 100 fs, 7 mJ per pulse, 1 kHz repetition rate). Broadband probe pulses were generated by focusing a portion of the Ti:sapphire output onto a sapphire crystal or YAG crystal, producing light spanning 350–1,550 nm. For short-time measurements (500 fs to 7 ns), a commercial spectrometer (HELIOS, Ultrafast Systems) was used, with probe ranges of 350–800 nm and 750–1,600 nm. Long-time measurements (1 ns to 1 ms) used probe ranges of 410–750 nm and 850–1,600 nm. A computer-controlled motorized delay stage was used to vary the probe path length. The pump beam was modulated at 500 Hz using a chopper, generating alternating probe pulses with and without excitation. Both beams were focused to an approximately 0.5-mm2 spot on the sample. The instrument response function was approximately 200 fs.
Femtosecond sum-frequency upconversion spectroscopy
PL decay kinetics within a 7-ns window were recorded using a femtosecond sum-frequency upconversion apparatus (HALCYONE, Ultrafast Systems) powered by a regenerative Ti:sapphire amplifier (Coherent; 800 nm, 100 fs, 7 mJ per pulse, 1 kHz repetition rate). The 800-nm beam was split: one portion pumped an optical parametric amplifier to produce tunable excitation pulses and the other served as the gate pulse. Emission from the sample was collected and co-focused with the 800-nm gate pulse onto a barium metaborate crystal, generating an upconverted signal by means of sum-frequency generation. This signal was passed through a 300-mm monochromator and detected by a spectrometer, providing a temporal resolution of 250 ps. All measurements were conducted on samples sealed in 2-mm airtight cuvettes, placed in a nitrogen-filled glovebox under continuous agitation.